LABS/Q® - Details
Architecture - LABS/Q®
The architecture of the LABS/Q® laboratory software also allows you to use the system in your specific infrastructure. With more than 30 years of experience and continuous optimization of the LIMSLABS/Q® we have ensured to offer a versatile and multi-layered laboratory software.
Our LIMS LABS/Q® is designed so flexibly that it can be easily adapted to the individual infrastructure of your laboratory, making it particularly user-friendly.
The following aspects of the system facilitate integration into your infrastructure:
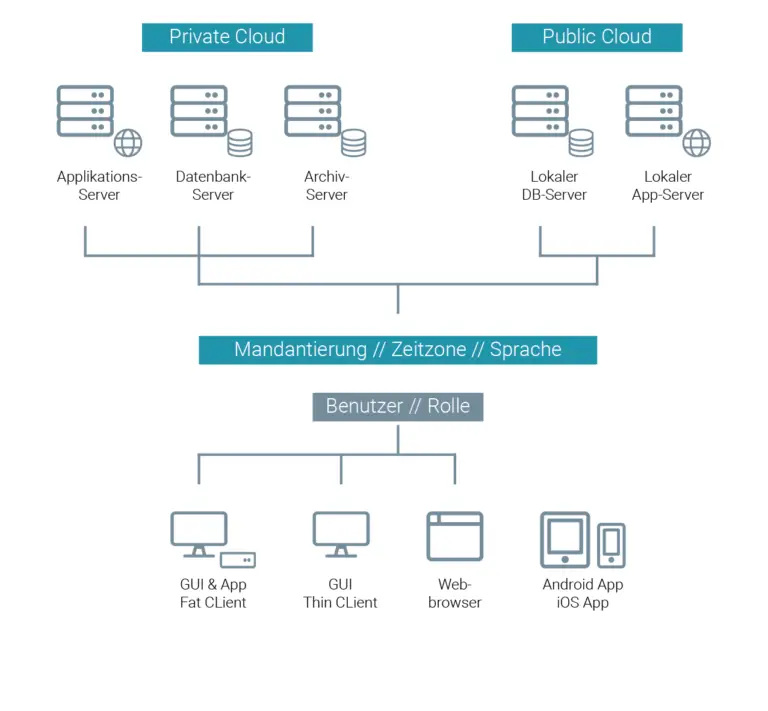
- Multi-tier application
Due to a consistent distribution of the application, the system can be used in any infrastructure. Thus, the database, business logic, graphical user interface (GUI server/client) and other parts, such as a help system, web services, interfaces, etc. are completely separated from each other. They can be installed on a single computer or in a distributed computer network.
Cloud capability - The LABS/Q® Lab software can also be operated as a private cloud or public cloud, such as SAP HCP (HANA Cloud Platform) as well as a hybrid cloud.
- Operating system independence
The JAVA-based development can be used on all common operating systems.
- Database independence
In principle, all SQL databases can be connected via the JDBC interface. Currently the products Oracle, MS SQL Server as well as HANA DB are supported. - DMS for unstructured data
Together with the standard system, the free content repository Apache Jackrabbit is also installed. Unstructured data, such as documents, regulations, reports, evaluations, images, raw data, etc., can thus be managed manually and automatically, in parallel to the objects of the LIMS LABS/Q®. Of course, other DMS/SDMS applications, such as Open Text, OpenLAB ECM, etc., can be connected instead of Jackrabbit.
Adaptability
The configurable "out-of-the-box" laboratory system
Every industry, every customer and even every laboratory has individual requirements for laboratory software. Therefore, the LIMS LABS/Q® offers extremely extensive basic functionalities and a high configurability of database, masks, report templates and functions.
Software customizability without consequences
All individual adaptations and changes to the system are seamlessly logged and documented. Since these are implemented without changing the standard source code, the update and upgrade capability of the laboratory software is maintained.
Master data management with LABS/Q®
Laboratory software for simple master data maintenance
All existing master data can be automatically imported, changed and deleted or cancelled via interfaces. Of course, manual maintenance is also possible. Via multi-level versioning with document control - supported by audit trail - every change in the LIMS LABS/Q® is completely traceable.
Document Management with LABS/Q®
The management module for your laboratory
With the integrated document management system - DMS for short - all your unstructured data, such as documents, reports, comments, images, raw data (also in AnIML format = Analytical Information Markup Language), etc., for all LABS/Q® objects can be stored manually or automatically, retrieved and found via a full text search.
LABS/Q® Laboratory Software Interfaces
Based on the standard interfaces of LABS LIMS, various uni- and bidirectional interfaces to measuring devices and external systems, such as ERP, MES, PLS, PLM and CDS have been developed. Their scope is constantly expanding, both as a result of developments by iCD. and by third parties. Industry standards, such as AnIML, are also supported. The following is a brief overview:
- SAP interface (individually configurable; certified by SAP)
- CDS interface to Thermo Fisher ChromeleonTM (also with raw data in AnIML format)
- CDS interface to Agilent OpenLab ChemStation
- CDS interface to Agilent OpenLab EZChrom
- CDS interface to Shimadzu LabSolutions
- Interface to Metrohm tiamoTM
- Interface to scales of the companies Mettler, Sartorius, Kern, etc.
- And much more
Scope of functions - LABS/Q®
Comprehensive functionality based on our many years of experience
With the purchase of the LABS/Q® laboratory information software, the functionality of all included modules is also available to the user. User roles can be used to define in detail which modules, functionalities and data can be accessed by the respective user. The functional scope of LABS/Q® LIMS is shown below:
- Durability and stability tests
- Environmental / hygiene monitoring
- Actions and measures (e.g. OOS management)
- Inspection equipment monitoring
- Analytical Quality Assurance AQA
- Batch management (batch tracing)
- Optimization of chemical syntheses or formulations
- LABSGraphic (control charts, statistical evaluations)
- Tablet APP for results recording and test order processing
- Interfaces to measuring devices and measuring systems as well as external applications, such as ERP, MES, DCS, etc.
LABS/Q® - Test Order Processing Laboratory Information Software
Support by LABS/Q® in processing your test orders
Test orders can be created manually as well as automatically via interfaces or internal modules (e.g. scheduling). Thereby, various status changes can be passed through during the test order processing. After completion of processing, these test orders are automatically archived by the LABS/Q® laboratory information software. To support the processing, in addition to various options for manual measurement value acquisition, automatic acquisition from the connected measuring devices is also enabled (see Interfaces). An integrated LES functionality (Laboratory Execution System) supports the employee during the test order processing. A special APP for tablets is also offered for test order processing (see APP). Of course, an ELN (Electronic Laboratory Notebook), such as OpenLab ELN from Agilent, can also be connected.
The LABS/Q® LIMS modules
RFID (Android only), image capture, LES functions, Google Maps to display sampling locations, and voice capture are supported. This APP can also work offline (without connection to the LIMS) with the data previously transferred from the LIMS and capture measured values, images and comments. If a connection to the LIMS is available again later, then the data is automatically synchronized. Thus, the APP can also be used outside the laboratory without network access.
LABSGraphic
LABSGraphic is available as a tablet app for LABS/Q® LIMS. This app can also be used independently of LABS/Q® LIMS in offline mode. Data can be imported from LABS/Q® LIMS and evaluated and compared graphically and statistically. Thus, e.g. process data can be evaluated together with quality data and dependencies can be detected if necessary.
Durability and stability tests
These kinds of tests can be performed in LABS/Q®, from planning, storage, sampling and testing of samples, to graphical and statistical evaluation of the results. This includes e.g. shelf life predictions and trends, shelf life extrapolations and thermal stress tests (Arrhenius), regression calculations, etc., from stress tests on developmental stabilities to "on-going" and "follow-up" stability tests during production.
Actions and measures
These modules and the functionalities they contain enable individual definition of workflows for a wide variety of actions, such as OOS/OOT (Out of Specification, Out of Trend) and audits. Associated actions, such as root cause analysis, retesting, etc., can also be planned, processed and evaluated.
Recipes
LABS/Q ® includes a solution for recipe development and recipe optimization. The goal of recipe optimization is to reduce manufacturing costs and optimize resource consumption, thus increasing the sustainability of production processes.
Environmental / hygiene monitoring
Production-accompanying and scheduled tests at 1 to n sample points can be triggered automatically by so-called project orders. A group report function in connection with LABSGraphic enables a comfortable, graphical as well as statistical evaluation of the data.
Test/measurement equipment monitoring
In addition to the management of test equipment and measuring equipment batches, automatic qualification, calibration and system suitability of the test equipment or systems can also be carried out and graphically and statistically evaluated (e.g. test equipment capability).
Get into conversation
Is your interest aroused? Do you still have questions? Do you need more information? Are you looking for a conversation? We are looking forward to the dialog with you!